Counterfeiters threaten $1 billion pipeline of medicine.
You are your company’s brand protection manager. Counterfeit copies of one of your major pharmaceutical brands turn up in the U.S. market. You have no security measures in place to allow patients or inspectors to tell the real stuff from the fake. Consequently, $1 billion worth of your product, already in the distribution pipeline, can’t be sold—at least not until you come up with some method of allowing patients and retailers to verify that your medicine is actually authentic.

Patient safety, your company’s hard-earned reputation not to mention $1 billion in sales are all under severe threat. Time to send out a SOS.
This is exactly the situation one of our customers found themselves in. And when they came to us for help, Authentix answered the call. Our customer desperately needed a way to instantly authenticate medicines in the field. No problem, we said.
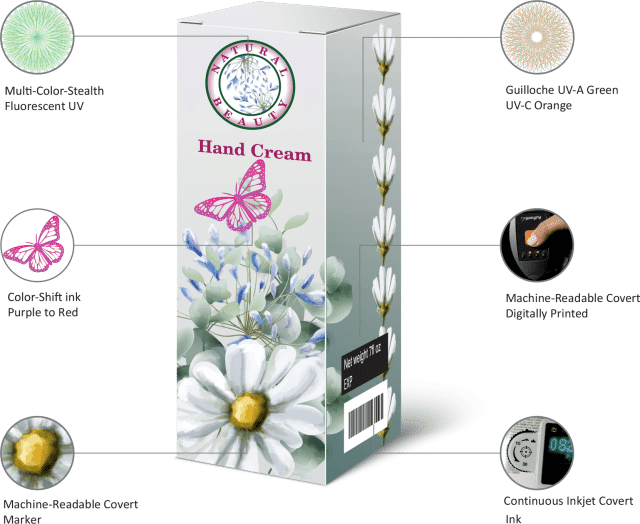
We immediately jumped into action. The customer’s product was repackaged to include a variety of authentication features that could be identified by patients and inspectors, both in the field and in the laboratory. These included:
- Overt, color-shifting inks can be readily distinguished by patients.
- Covert, machine-readable inks can be detected in the field by inspection staff with appropriate readers.
- Forensic markers can only be detected under laboratory analysis.
A happy ending
The Authentix solution to our customer’s counterfeiting problem provided a secure means of instantly identifying authentic from counterfeit medicines. The benefits were immediate and significant:
- $1 billion worth of product frozen within the supply chain was released for sale
- The expense of a full product recall was averted
- The customer was able to mitigate the risk of potential lawsuits
- And, our customer’s brand protection manager instantly became the organization’s authentication “superhero.”
Most importantly, confidence in the brand was restored among physicians, pharmacists and patients.
Every superhero needs a partner.
What would Batman be without Robin? The Green Hornet without Kato? Not much, if you ask us. If you want to be your company’s authentication superhero, contact us today. In true superhero style, Authentix is the partner with the innovative solution to boost your authentication powers!
Download PDF Here







 Whitepaper – Protecting Critical Care Drugs
Whitepaper – Protecting Critical Care Drugs